Feedback Feedback
Amazingly, in 6 years of
experimentation, not one credible case of toxicity or adverse reaction has
been observed. These are preliminary comments and nothing contained herein
should be construed to be conclusive and/or positively curative.
**

How
Can It
Make So Much?
 We have been asked a number of times how our 8 fl. oz. bottle of
AO Alkalizer can possibly make so much finished product (i.e. 7 to 10
gallons at pH 1.6 to 2.0). The answer is surprisingly simple: it really is
that concentrated. When you get your product, you can test it yourself.
The concentrate will be in the 0.0 to 0.5 range. Since finished product is
usually in the 1.6 to 2.0 range, you just have to remember your high
school chemistry: namely, that pH is the negative logarithm of the
hydrogen ion. So a "0" is 10 times as acidic as a "1," a "1" is 10 times
as acidic as a "2" ... so a "0" is 100 times stronger than a "2." This is
why you can dilute our concentrate at ratios above 1:100 and you still
have a strong acid solution that is under 2.0. When you get your Kit you
can experiment and see this for yourself!
Top of Page
|
A major
discovery . . .



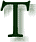 he hydronium ion has been known about for some time. The very measure
used to quantify acidity and alkalinity is, by definition, based on the
negative logarithm (pH) of the reduced version of this fundamental
chemical, and the entity itself has
been well defined as has been the method by which ordinary chemical
acids increase
hydronium ions in water. Moreover, we know the concentration
of hydronium ions in blood plasma, measured in nanomoles/Liter. he hydronium ion has been known about for some time. The very measure
used to quantify acidity and alkalinity is, by definition, based on the
negative logarithm (pH) of the reduced version of this fundamental
chemical, and the entity itself has
been well defined as has been the method by which ordinary chemical
acids increase
hydronium ions in water. Moreover, we know the concentration
of hydronium ions in blood plasma, measured in nanomoles/Liter.
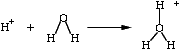  It seems bizarre, therefore, to suggest that it
has taken us until the 21st century to figure out how to make a
stabilized, highly concentrated version of hydronium ions in solution, and uncover
its medicinal uses. But bizarre or not, this just happens to be the
case. If you take our "AO Alkalizer" with a very acidic measure of
under pH 0.5, and you test it using spectrometry, chromotography, or any
other analytical chemical measure (other the pH), what do you get? You
would appear (though erroneously, read Stability) to have nothing more than an aqueous
solution of diluted sulphuric acid ("normal 1.8" or about 10:1 dilution
ratio) -- except this is a solution you can easily swallow (more
comfortably if diluted to a pH of 2.0, granted) at acidic levels, which,
if we were talking about any other acid in the 0.0 to 0.5 pH range, would
have potentially severe health consequences. (In Shelf-Life Considerations we talk about how the
product is made, which we recommend you read.) It seems bizarre, therefore, to suggest that it
has taken us until the 21st century to figure out how to make a
stabilized, highly concentrated version of hydronium ions in solution, and uncover
its medicinal uses. But bizarre or not, this just happens to be the
case. If you take our "AO Alkalizer" with a very acidic measure of
under pH 0.5, and you test it using spectrometry, chromotography, or any
other analytical chemical measure (other the pH), what do you get? You
would appear (though erroneously, read Stability) to have nothing more than an aqueous
solution of diluted sulphuric acid ("normal 1.8" or about 10:1 dilution
ratio) -- except this is a solution you can easily swallow (more
comfortably if diluted to a pH of 2.0, granted) at acidic levels, which,
if we were talking about any other acid in the 0.0 to 0.5 pH range, would
have potentially severe health consequences. (In Shelf-Life Considerations we talk about how the
product is made, which we recommend you read.)
 Alpha Omega Labs is in a partnership
involving the manufacture of AO Alkalizer for the food, beverage, and
pharmaceutical markets, and we are introducing it to the general public. Although there is far more about
AO Alkalizer that scientists
don't know than what we do know -- this much is certain: after 4 years of
testing, we have found our Alkalizer solution to be completely non-toxic and
hypoallergenic -- in fact, it has been used extensively at the pH 2.0
dilution value to treat eye infections! The researchers with whom we are
associated have been gargling with it (at pH 2.0 dilution) for years --
ever since they learned that, for some reason, they stopped getting any
plaque buildup on their teeth when they used it orally. Alpha Omega Labs is in a partnership
involving the manufacture of AO Alkalizer for the food, beverage, and
pharmaceutical markets, and we are introducing it to the general public. Although there is far more about
AO Alkalizer that scientists
don't know than what we do know -- this much is certain: after 4 years of
testing, we have found our Alkalizer solution to be completely non-toxic and
hypoallergenic -- in fact, it has been used extensively at the pH 2.0
dilution value to treat eye infections! The researchers with whom we are
associated have been gargling with it (at pH 2.0 dilution) for years --
ever since they learned that, for some reason, they stopped getting any
plaque buildup on their teeth when they used it orally.
 Although our AO Alkalizer will not harm ordinary skin (for
reasons that are still baffling the academics with whom we have shared
it), it will burn holes in fabrics (particularly cotton), in its undiluted
concentrate form, after a day or two. Since we sell the concentrate
version, please be well aware of this. We discovered this for ourselves
after asking why those in the lab had holes in their work clothes. You should not
interpret any of our lab notes as an indication of a claimed treatment or
cure. We doubt that any regulatory agency on earth will ever approve it as
a claimed treatment. Although our AO Alkalizer will not harm ordinary skin (for
reasons that are still baffling the academics with whom we have shared
it), it will burn holes in fabrics (particularly cotton), in its undiluted
concentrate form, after a day or two. Since we sell the concentrate
version, please be well aware of this. We discovered this for ourselves
after asking why those in the lab had holes in their work clothes. You should not
interpret any of our lab notes as an indication of a claimed treatment or
cure. We doubt that any regulatory agency on earth will ever approve it as
a claimed treatment.
|
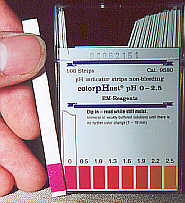
 Using pH strips that you can buy from a local drug store,
you will see that our H3O concentrate consistently tests at 0.0 to 0.5.
And yet... it is completely non-corrosive. Using pH strips that you can buy from a local drug store,
you will see that our H3O concentrate consistently tests at 0.0 to 0.5.
And yet... it is completely non-corrosive.
Label
Information
INGREDIENTS: Purified water,
high concentration hydronium ions in aqueous solution, sulphuric acid.
DIRECTIONS: Use the pH strips that accompany this product to
lower acidity to desired level. Since your base water will probably be on
the acid side of a neutral 7.0, you will need to initially experiment to
see what ratio of water-to-Alkalizer produces the desired pH product you are
looking for. The pH you are looking for will depend on your particular
application. Since AO Alkalizer has numerous uses, you will want to have several
dilution ratios to serve different applications for your hydronium. We
provide some examples on the right side panel of this label.
DILUTION GUIDELINES: If you are using our Alkalizer concentrate for
the first time, use pH strips to find
the dilution ratios that match your “base water.” Since 1 fl. oz. of
Alkalizer to a gallon of water will achieve a 1.6 to 2.0 pH using most purified
water (1:129 ratio), many people use this for most applications. If you
are using the product for certain medical applications or for your own
research at more acidic ranges, you may want a more precise pH. Your base
water is ideally “purified” (distilled or “RO”).
WARNING: If
you get Alkalizer concentrate in your eyes, just wash/dilute with water.

The New
"Super"
Preservative?
 Preliminary testing shows that AO Alkalizer has incredible potential as a
food preservative. The same "super anti-microbe" characteristics it has
generated in medical applications is manifesting in food preservation
tests. In a separate report, AO Alkalizer & Food
Stability: A Test, we discuss test results obtained last year on
post-harvest tomatoes, which we hope will encourage other,
politically-neutral research institutions to follow our lead and those of
our associates. If the results to date stand up under scrutiny, there is
the distinct possibility that stabilized AO Alkalizer could not only replace the
usual standard-fare food-grade acidulents, such as phosphoric, citric, and
lactic acids in a variety of applications, but, of even greater
significance and importance, it would replace entire categories of
preservatives: virtually all sorbates, benzoates, proprionates, nitrates,
and their salts for most food processing stability applications,
particularly in fluid and "IM mode" (intermediate moisture) substrates.
|
** DISCLAIMER: The
information on this page is for educational purposes, and to act as
a guide to prospective researchers. AO Alkalizer is not intended
for use in the diagnosis, treatment, cure, mitigation, or prevention
of any disease, or to affect the structure or bodily function, of
man or other
animals.
|
|
 Feedback
Feedback